 |
 |
For most of history, visible light was the only known part of the electromagnetic spectrum. The ancient Greeks recognized that light traveled in straight lines and studied some of its properties, including reflection and refraction. In 1666, Sir Issac Newton observed that the spectrum of colors exiting a prism creates a rainbow. By 1670 he demonstrated that the multicolored spectrum produced by a prism could be recombined into white light by a lens and a second prism.
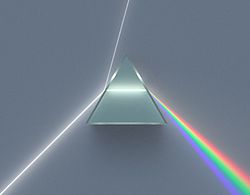
The dispersion of white light through a prism
Today we now know that visible light is only a small portion of what is called the electromagnetic spectrum. Continue to learn more below and also click on this companion learning source from NASA.
The first discovery of electromagnetic radiation other than visible light came in 1800, when William Herschel discovered infrared radiation. He was studying the temperature of different colors by moving a thermometer through light split by a prism. He noticed that the highest temperature was beyond red. He theorized that this temperature change was due to "calorific rays" which would be in fact a type of light ray that could not be seen. It wasn't until the latter 20th century when we could build imaging infrared detectors to view the universe. The infrared region of the spectrum is divided into near-, mid-, and far-infrared.
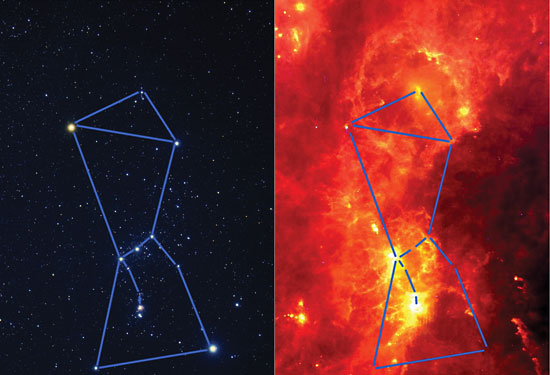
The constellation of Orion seen in visible and infrared light

The Orion Nebula in infrared and visible light
Molecules tend to absorb infrared energy causing them to vibrate based upon their atomic structure. In space, there are many regions which are hidden from optical telescopes because they are embedded in dense regions of gas and dust. However, infrared radiation, having wavelengths which are much longer than visible light, can pass through dusty regions of space without being scattered. This means that we can study objects hidden by gas and dust in the infrared, which we cannot see in visible light, such as the center of our galaxy and regions of newly forming stars. Many objects in the universe which are much too cool and faint to be detected in visible light, can be detected in the infrared. These include cool stars, infrared galaxies, clouds of particles around stars, nebulae, interstellar molecules, brown dwarfs and planets.
Infrared light is absorbed at many wavelengths by water vapor in the Earth's atmosphere, so most infrared telescopes are at high elevations in dry places, above as much of the atmosphere as possible. NASA operates infrared telescopes in orbit such as WISE and event has an aircraft equipped with infrared telescopes called SOFIA. Slightly more than half of the total energy from the Sun arrives on Earth in the form of infrared light. The balance between absorbed and emitted infrared radiation has a critical effect on Earth's climate.
Did You Know? We can feel infrared light as heat but cannot see it. Heat lamps at a fast food restaurant that keep your french fies warm give off most their light in the infrared.
Ultraviolet
In 1801, the German physicist Johann Wilhelm Ritter made the hallmark observation that invisible rays just beyond the violet end of the visible spectrum darkened silver chloride-soaked paper more quickly than violet light itself. He called them "oxidizing" or "chemical" rays to emphasize chemical reactivity and to distinguish them from "heat rays", discovered the previous year at the other end of the visible spectrum.
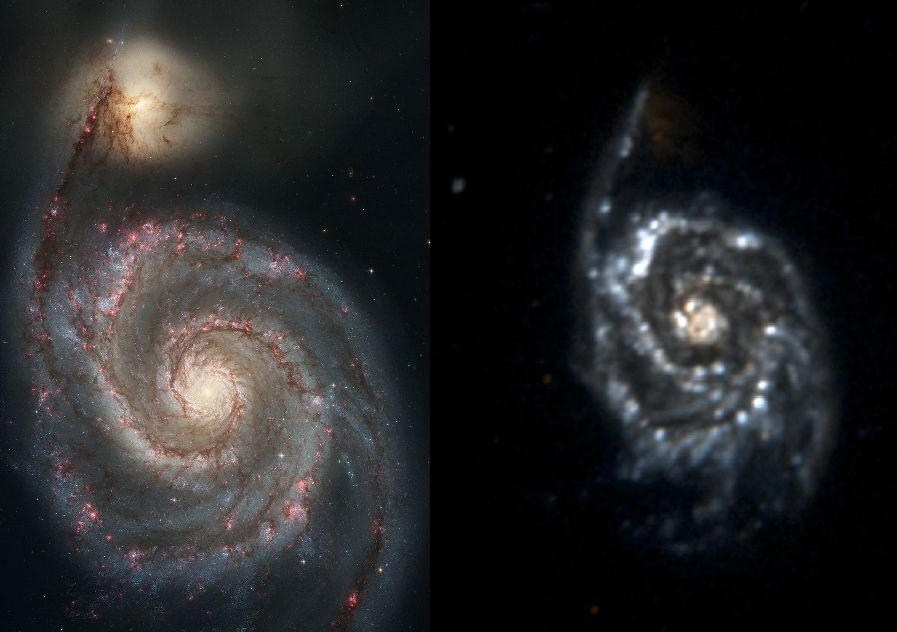
Messier 51 in visible and ultraviolet light
Very hot objects emit UV radiation. In M51, young hot stars show up well in ultraviolet yet the companion galaxy shows no new star formation. In our solar system, the Sun is a source of the full spectrum of ultraviolet radiation, which is commonly subdivided into UV-A, UV-B, and UV-C. These are the classifications most often used in Earth sciences. UV-C rays are the most harmful and are almost completely absorbed by our atmosphere. UV-B rays are the harmful rays that cause sunburn. Exposure to UV-B rays increases the risk of DNA and other cellular damage in living organisms. Fortunately, about 95 percent UV-B rays are absorbed by ozone in the Earth's atmosphere.
In 1895 Wilhelm Röntgen noticed a new type of radiation emitted during an experiment with an evacuated tube subjected to a high voltage. He called these radiations x-rays and found that they were able to travel through parts of the human body but were reflected or stopped by denser matter such as bones. Before long, many uses were found for them in the field of medicine. The first attempt at X-ray astronomy was conducted at White Sands Missile Range in January 1949 when a detector was lofted by a V-2 suborbital rocket.

Messier 51 in visible and X-ray light
The Sun emits X-rays giving clues to the temperature of the corona. A live image of the Sun in X-rays can be seen on the AAG 'Planets' page. Look for the live images of the Sun and the one on the far right (yellow) is how our Sun looks in X-rays.
The last portion of the electromagnetic spectrum was filled in with the discovery of gamma rays. In 1900 Paul Villard was studying the radioactive emissions of radium when he identified a new type of radiation that he first thought consisted of particles similar to known alpha and beta particles, but with the power of being far more penetrating than either. A discovery in gamma-ray astronomy came in the late 1960s and early 1970s from a constellation of military defense satellites. Detectors on-board the Vela satellites, designed to detect flashes of gamma rays from nuclear bomb blasts, began to record bursts of gamma rays from deep space rather than the vicinity of the Earth.
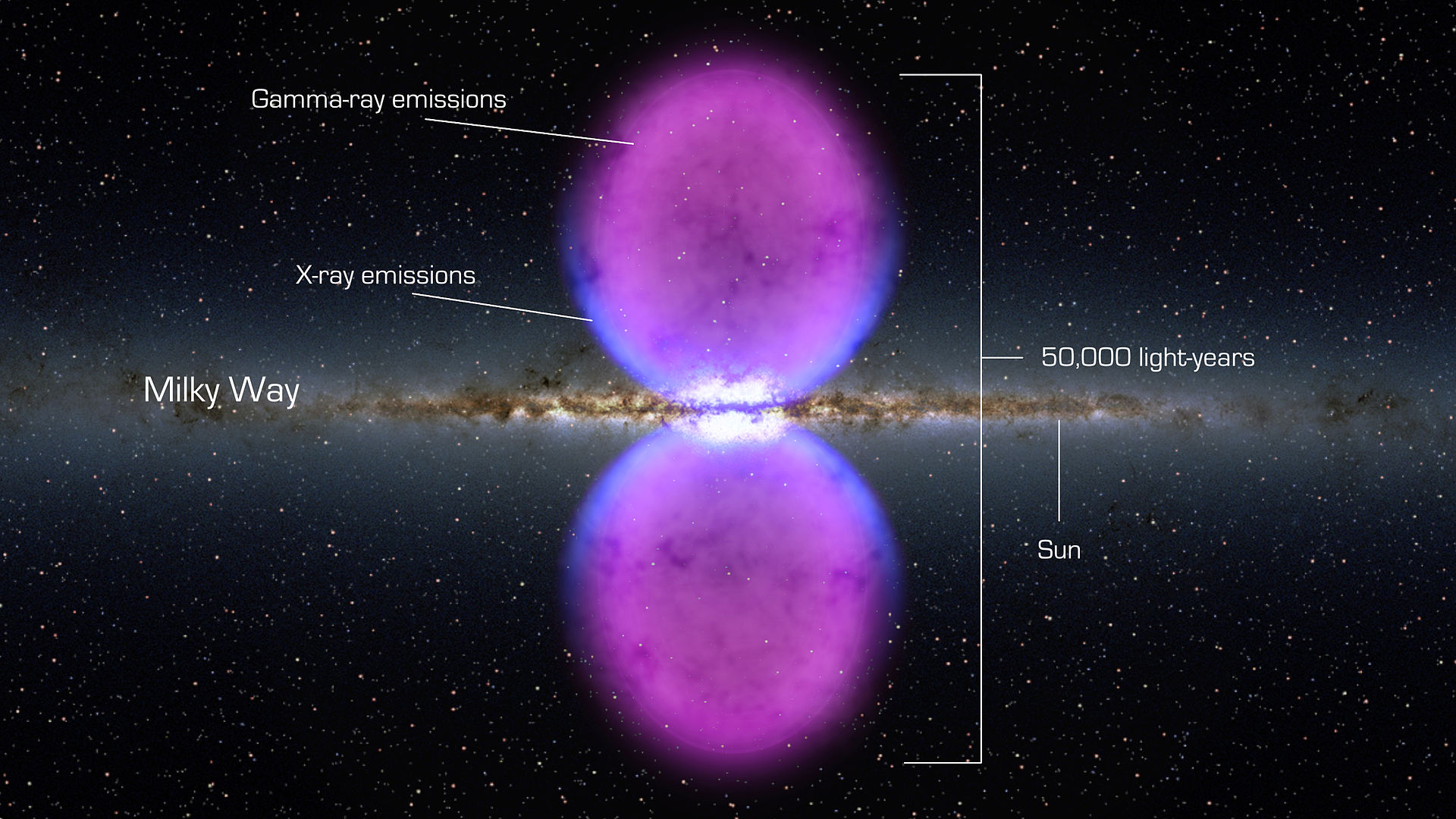
Recent observations by the Fermi Space Telescope hint at large gamma-ray bubbles eminating from our galactic core
Gamma rays have the smallest wavelengths and the most energy of any wave in the electromagnetic spectrum. They are produced by the hottest and most energetic objects in the universe, such as neutron stars and pulsars, supernova explosions, and regions around black holes. On Earth, gamma waves are generated by nuclear explosions, lightning, and the less dramatic activity of radioactive decay.
In the 1860's, Scottish scientist James Clerk Maxwell developed a scientific theory to explain electromagnetic waves. He noted that electrical fields and magnetic fields can couple together to form electromagnetic waves. The initial detection of radio waves from an astronomical object was made in the 1930s, when Karl Jansky observed radiation coming from the Milky Way. Subsequent observations have identified a number of different sources for radio emissions. These include stars and galaxies, as well as entirely new classes of objects, such as radio galaxies, quasars, pulsars, and masers. The discovery of the cosmic microwave background radiation, regarded as evidence for the Big Bang theory, was made through radio astronomy.
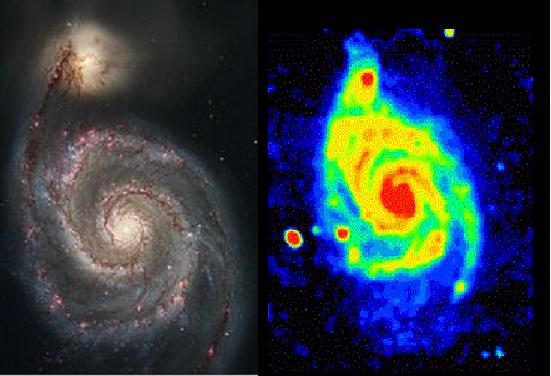
Messier 51 in visible and radio light
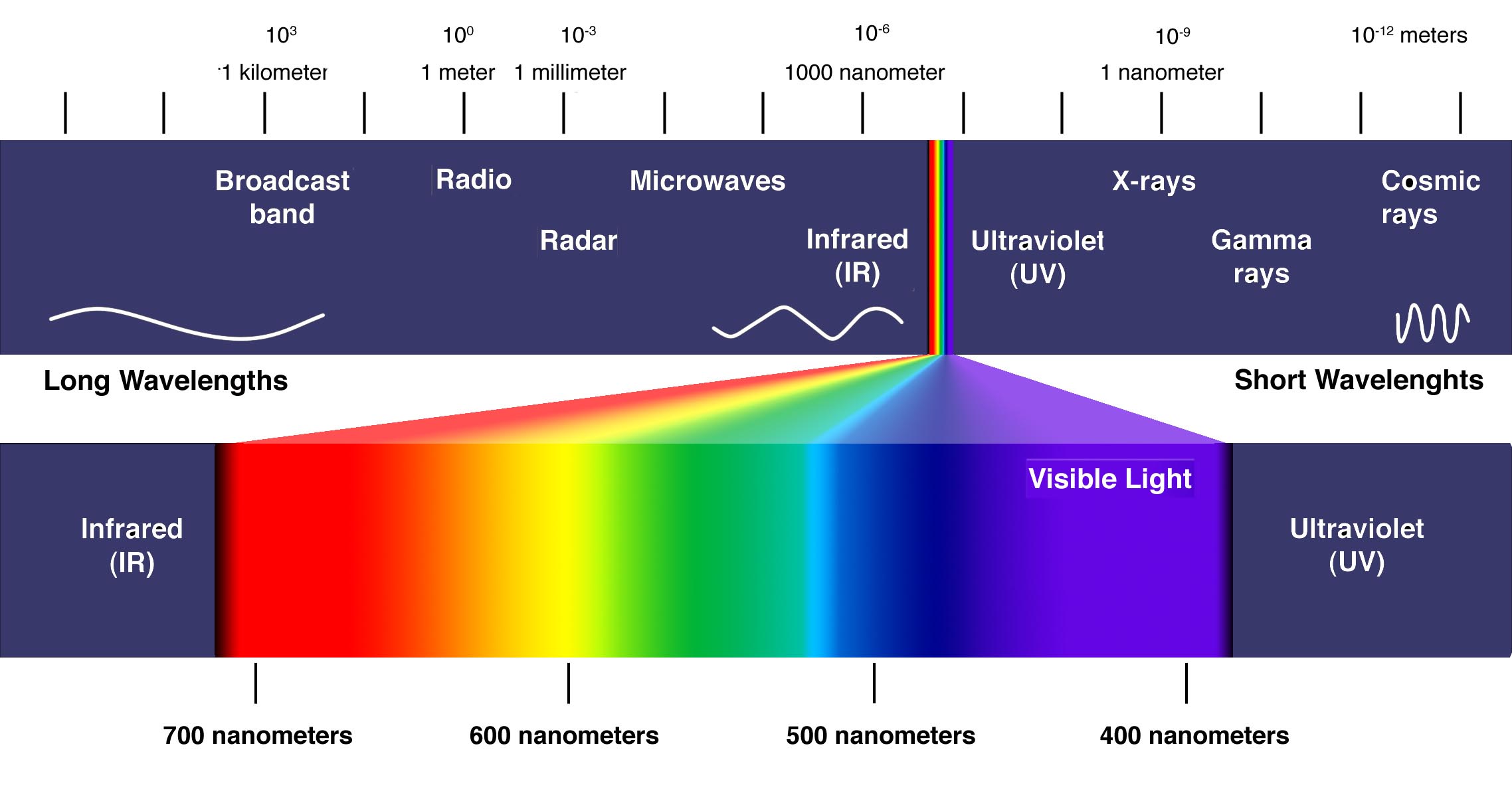
The Electromagnetic Spectrum
For centuries, astronomers have only examined the visible portion of the electromagnetic spectrum. The Earth's atmosphere effectively blocks low frequency radio waves, most infrared and ultraviolet light and all X-ray and gamma ray sources. Sorry Marconi, your radio signals are never going to be picked up by the aliens - the Earth's ionosphere reflected them back down to the surface.
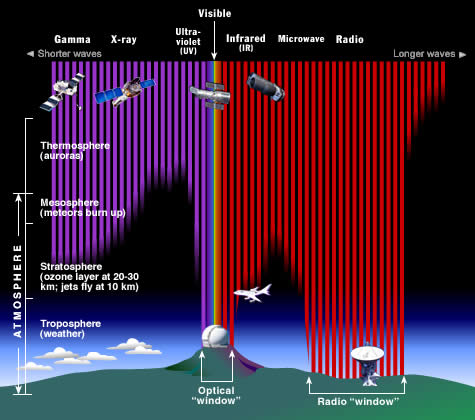
Filtering of the EM spectrum by Earth's atmosphere
The Universe sends us light at all wavelengths of the electromagnetic spectrum. However, most of this light does not reach us at ground level here on Earth. Why? Because we have an atmosphere which blocks out many types of radiation while letting other types through. Fortunately for life on Earth, our atmosphere blocks out harmful, high energy radiation like X-rays, gamma rays and most of the ultraviolet rays. It also block out most infrared radiation, as well as very low energy radio waves. On the other hand, our atmosphere lets visible light, most radio waves, and small wavelength ranges of infrared light through, allowing astronomers to view the Universe at these wavelengths. Most of the infrared light coming to us from the Universe is absorbed by water vapor and carbon dioxide in the Earth's atmosphere. Only in a few narrow wavelength ranges, can infrared light make it through (at least partially) to a ground based infrared telescope.
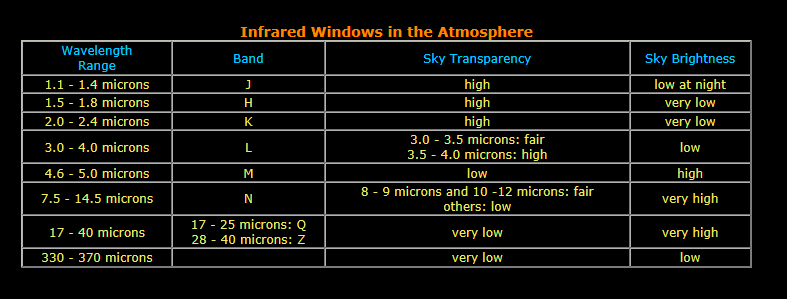
The Earth's atmosphere causes another problem for infrared astronomers. The atmosphere itself radiates strongly in the infrared, often putting out more infrared light than the object in space being observed. This atmospheric infrared emission peaks at a wavelength of about 10 microns (micron is short for a micrometer or one millionth of a meter). So the best view of the infrared universe, from ground based telescopes, are at infrared wavelengths which can pass through the Earth's atmosphere and at which the atmosphere is dim in the infrared. Ground based infrared observatories are usually placed near the summit of high, dry mountains to get above as much of the atmosphere as possible. Even so, most infrared wavelengths are completely absorbed by the atmosphere and never make it to the ground. From the table below, you can see that only a few of the infrared "windows" have both high sky transparency and low sky emission. These infrared windows are mainly at infrared wavelengths below 4 microns.
The earliest scientific theories of the nature of light were proposed around the end of the 17th century by Dutch astronomer Christian Huygens. He proposed a theory that explained light as a wave phenomenon. Sir Issac Newton, who had discovered the visible spectrum in 1666, held that light is composed of tiny particles, or corpuscles, emitted by luminous bodies. For more than 100 years, Newton's corpuscular theory of light was favored over the wave theory, partly because of Newton's great prestige and partly because not enough experimental evidence existed to provide an adequate basis of comparison between the two theories. However, the wave theory fell back into favor with from the electromagnetic theory of James Clerk Maxwell (1864), who showed that electric and magnetic fields were propagated together and that their speed was identical with the speed of light.
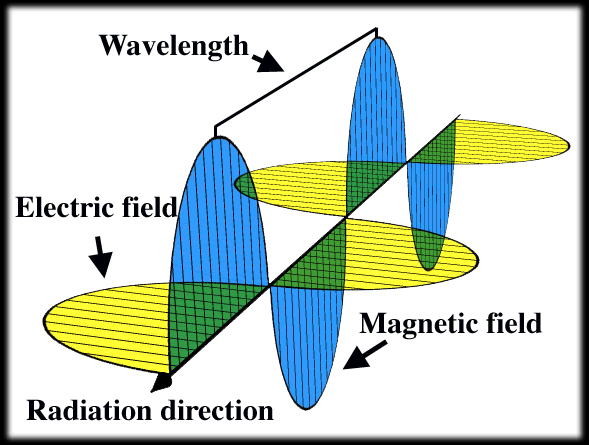
The Electromagnetic Wave
Mawell showed that the electric field is pushed along by the perpendicular magnetic field, which in turn pushes the electric field along and the propagation occurs at 300,000 meters per second. It thus became clear that visible light is a form of electromagnetic radiation, constituting only a small part of the electromagnetic spectrum. Maxwell's theory was confirmed experimentally with the discovery of radio waves by Heinrich Hertz in 1886.
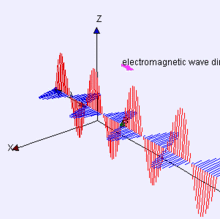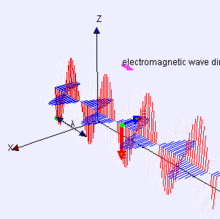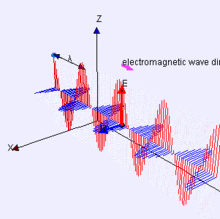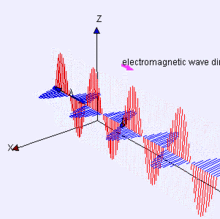
Propagation of the EM Wave
In 1905, Einstein extended the quantum theory of thermal radiation proposed by Max Planck in 1900 to cover not only vibrations of the source of radiation but also vibrations of the radiation itself. He thus suggested that light, and other forms of electromagnetic radiation as well, travel as tiny bundles of energy called light quanta, or photons. According to our understanding today, the electromagnetic field itself is produced by photons, which in turn result from a local (gauge) symmetry and the laws of quantum field theory.
As soon as astronomers understood the nature of light and could build sensors to detect various forms of the electromagntic spectrum, this opened up the universe's true nature. To learn about how astronomers can determine the nature of objects across the light years, click here: Decoding Cosmic Spectra
|